Regulation and Physiology of Menstrual Cycle
The menstrual cycle is necessary for reproduction. The normal menstrual cycle is an orderly cyclic change that occurs in the ovary for the production of eggs and in the uterus for the preparation of the uterus for pregnancy. When pregnancy fails, menstruation occurs. Menstruation is a cyclic sloughing of the uterine lining in response to the interactions of hormones produced by the hypothalamus, pituitary, and ovaries.
Reproductive Neuroendocrinology
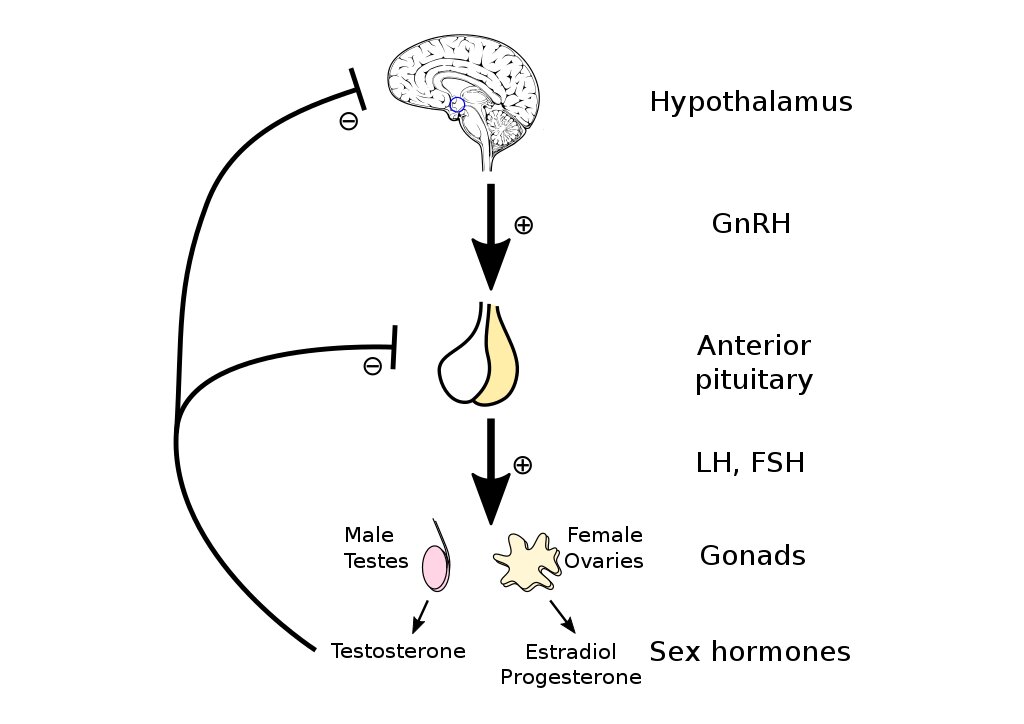
The hypothalamic-pituitary function along with the ovarian function is essential for maintaining a normal menstrual cycle.
- In response to peripheral and central nervous system signals, the Hypothalamus secrets the neurotransmitter Gonadotropin-releasing hormone (GnRH) which acts on the pituitary
- In response to GnRH, the Pituitary secretes follicle-stimulating hormone (FSH) and luteinizing hormone (LH) which acts on the ovary
- FSH and LH acts on ovaries to produce estrogen and progesterone
It is these GnRH, FSH, LH, estrogen, and progesterone that are predominantly involved in the regulation of the menstrual cycle.
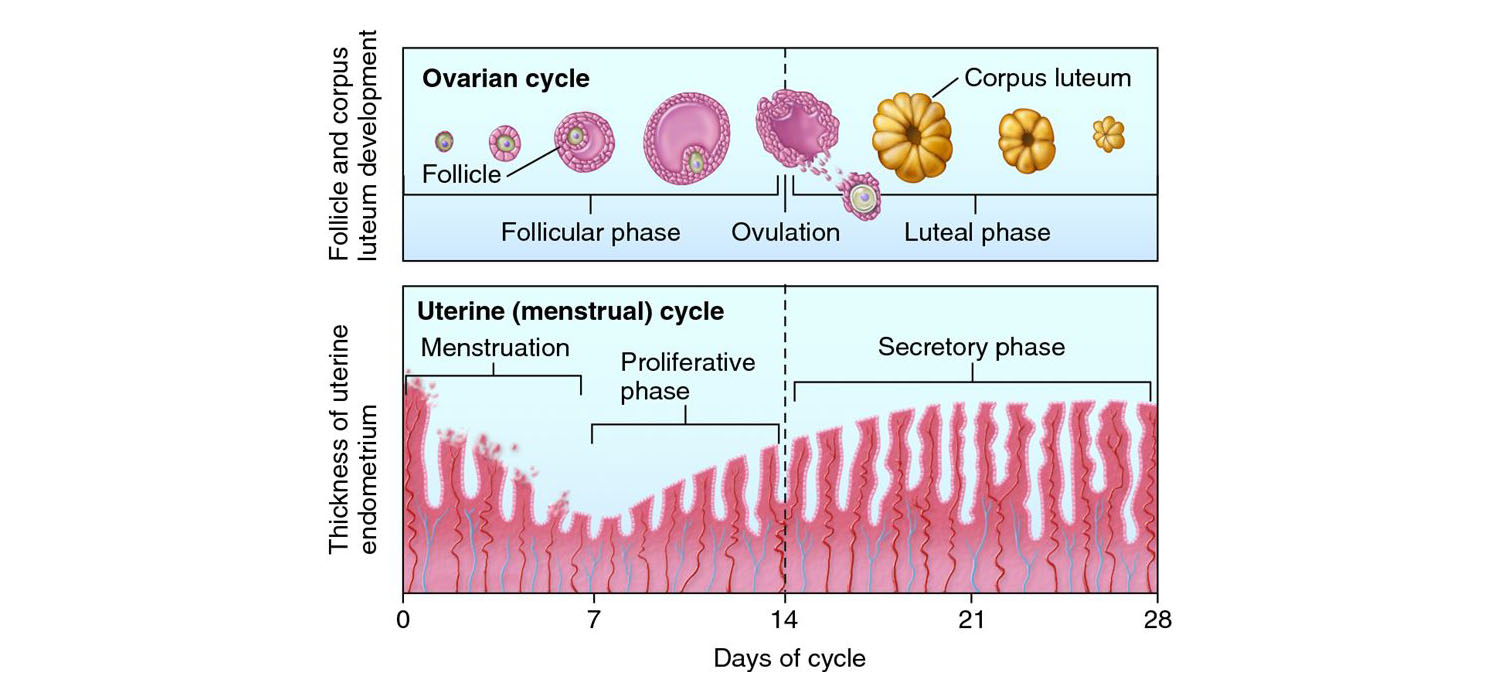
Menstrual cycles and phases
The menstrual cycle in humans is a monthly cycle that averages 28 days per cycle. There is inter and intra-individual variation in the cycle length. It is counted from the first day of menstrual bleeding. Based on the events that take place, the ovary and endometrium each have different phases.
The phases of the ovarian cycle are
- Follicular phase
- Ovulation
- Luteal phase along
- Luteal-follicular transition phase.
The phases of the uterine endometrium cycle are
- Menstruation
- Proliferative phase
- Secretory phase
Ovarian cycle
It requires a sequence of events and actions of hormones and autocrine-paracrine peptides on the follicles from the primordial follicle through the stages of the preantral, antral, and preovulatory follicle.
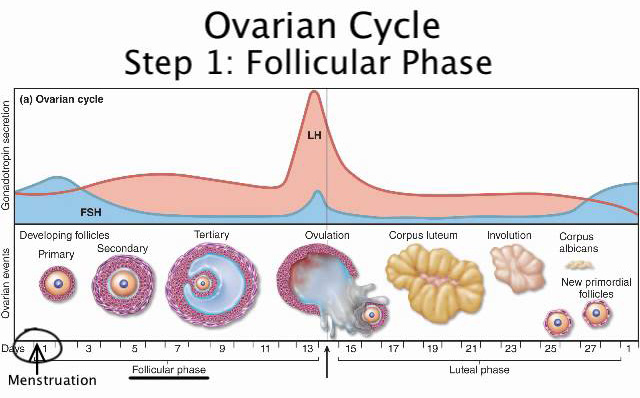
I. Follicular phase
It starts from 1st day of menstruation until ovulation. The primary goal is to develop a viable follicle capable of undergoing ovulation. The early events of the follicular phase are initiated by a rise in FSH levels at the luteal-follicular transition which causes the rescue of a cohort of preantral follicles from apoptosis – a phenomenon called recruitment. For recruitment to occur, a certain minimum concentration of FSH is required which is called FSH threshold. The time frame for which the FSH levels need to be maintained for follicular recruitment and growth is called the FSH window. The stages of follicular growth through various stages are as follows.
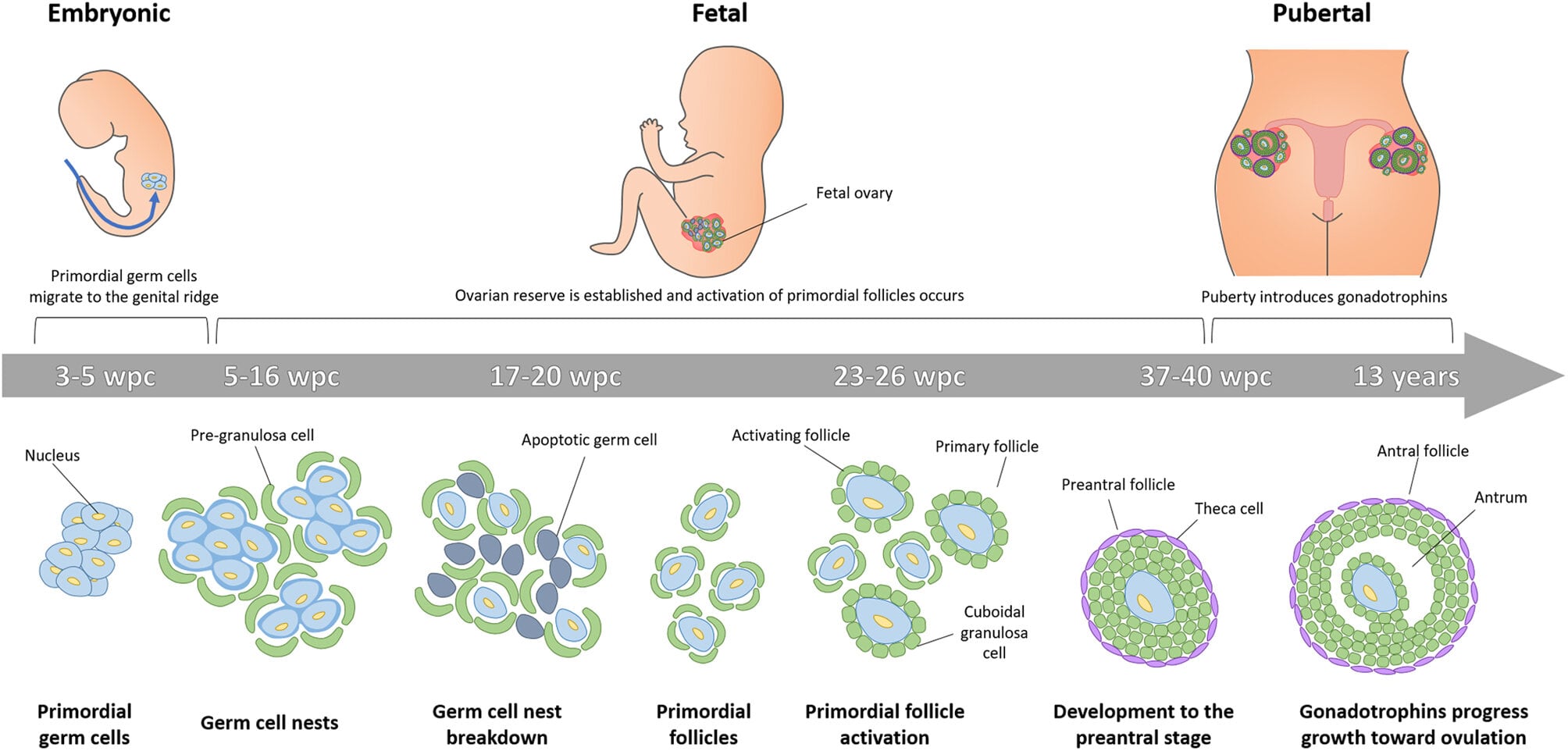
1. Primordial follicle
Consists of oocytes in the diplotene phase of the meiotic prophase surrounded by a single layer of spindle-shaped granulosa cells. The primordial follicle gets stimulated by nutrition and other factors such as energy, oxygen, etc. which results in the conversion of spindle shaped granulosa cells to cuboidal cells.
2. Primary follicle
The cuboidal cells undergo multiplication and the granulosa cells get separated from the stromal cells by a basal lamina. The stromal cells are further divided into theca interna and theca externa. Recruitment is the process by which the cohort of follicles responding to FSH at the beginning of the cycle gets rescued from apoptosis and competes for the selection of the dominant follicle. Follicular atresia is regulated by FSH, LH, IGF-1, EGF, BFGF, TNF-alpha, IL-6and others.
3. Preantral follicles
Accelerated granulosa cell growth results in multicellular proliferation which increases the production of estrogen, and also androgens and progestins. Oocyte enlargement occurs which is surrounded by zona pellucida (glycoprotein acellular membrane). There is an increase in FSH receptors which activates the FSH-dependent conversion of androgens to estrogen favouring the estrogen microenvironment. The fate of the preantral follicle: is in a delicate balance between androgen or estrogen dominance in the microenvironment.
Oocyte maturation and follicular antrum formation correspond to each other. When antrum formation occurs, the granulosa cells differentiate into two anatomically and functionally distinct lineages
- Mural granulosa cells that line the wall of the follicle
- Cumulus cells which are intimate with the oocyte. These cells have highly specialized transzonal cytoplasmic projections that penetrate through the zona pellucida and form gap junctions at their tips with the oocyte forming a cumulus-oocyte complex. The gap junction allows the passage of ions, metabolites, aa, cholesterol, and signaling molecules.
4. Antral follicle
This is the stage where the intercellular fluid between the granulosa cells eventually coalesces into a cavity called the antrum. The fluid in the antrum is rich in hormones, growth factors, and cytokines which nurture the oocyte. During the antral follicle stage, there is the selection of dominant follicles that secrete increasing amounts of estradiol. The synthesis of steroids is functionally compartmentalized within the follicle as the two-cell two two-gonadotropin system.
5. Preovulatory follicle
During the preovulatory stage, the oocyte proceeds in meiosis, approaching the completion of its reduction division. Approaching maturity, the preovulatory follicle produces increasing amounts of estrogen, peaking at 24-36 hours before ovulation. The onset LH surge occurs when peak levels of estradiol are reached.
II. Ovulatory phase
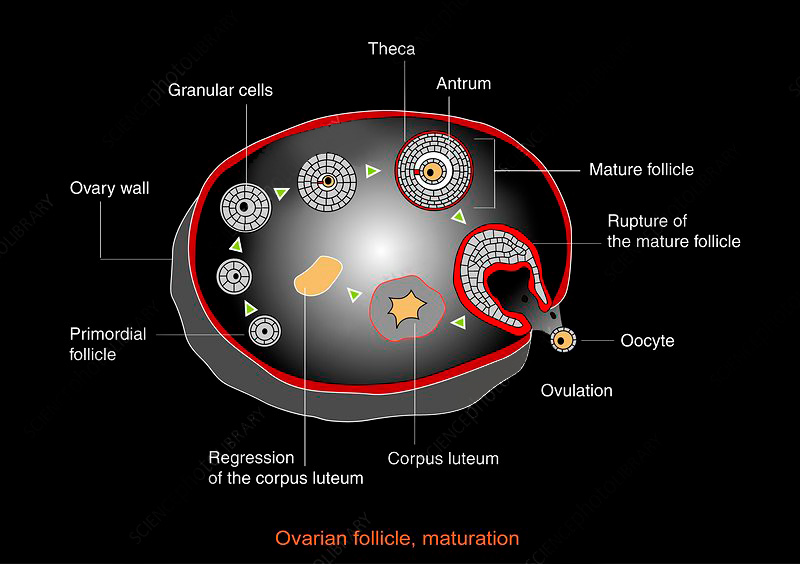
It is the phase of the menstrual cycle in which a mature egg is released from the ovarian follicle into the fallopian tube. The few days surrounding ovulation constitute the most fertile phase. Pulsatile GnRH secretion forms an important prerequisite for normal pituitary secretion. However, the feedback responses regulating gonadotropin levels are controlled primarily by ovarian feedback on the anterior pituitary cells. The onset of LH surge appears to be the most reliable indicator of impending ovulation, occurring 34-36 hours before follicle rupture. Ovulation occurs 10-12 hours after LH peak and 24-36 hours after estradiol peak.
III. Luteal phase
The luteal phase is defined by the luteinization of the follicles forming corpus luteum initiated by LH surge. There is the conversion of granulosa cells from predominantly androgen-converting cells to predominantly progesterone-synthesizing cells. Corpus luteum rapidly declines after 9-11 days after ovulation. If fertilization and subsequent implantation of the blastocyst do not occur, the CL undergoes apoptosis and becomes the corpus albicans, a white scar. If implantation occurs, the hCG from the pregnancy rescues the CL.
IV. Luteal-follicular Transition
It is a period of selective increase in FSH beginning about 2 days before the onset of menstruation. The increase in FSH is instrumental in rescuing approximately a 70-day cohort of follicles from atresia allowing a dominant follicle to begin its emergence.
Uterine Endometrial cycle
The cycle changes in the endometrium prepare for implantation in the event of fertilization and necessitate menstruation in the absence of fertilization. The endometrium consists of
- Functionalis layer that occupies the upper two-thirds layer. It is the site of proliferation and secretion meant for blastocyst implantation.
- Basalis layer serving as a source of regenerative endometrium after menstrual breakdown.
The functionalis layer is subdivided into the following two parts
- Stratum compactum- composed of dense stromal cells and gland necks
- Stratum spongiosum- contains predominantly endometrial glands, increased interstitial tissue, and less dense stroma.
The changes in endometrium are described in four phases which are explained as follows
I. Proliferative or follicular phase
The ovarian follicular phase corresponds to the proliferative phase. It spans from the end of menstruation until ovulation. During this phase, there is a proliferation of all elements slowly at first but later at a rapid pace. The proliferation peaks on days 8-10 of the cycle. The endometrial thickness by the end of the follicular phase is 12mm with a trilaminar pattern.
II. Secretory or luteal phase.
This phase starts at ovulation and lasts until the menstrual phase of the next cycle. The endometrial glands become tortuous and have increased secretory activity. Blood vessels undergo marked spiralling. Endometrial trilaminar pattern is lost.
III. Implantation phase
It comprises days 21-22 of the menstrual cycle. The endometrial glands become increasingly tortuous and the stroma becomes oedematous. The endometrium has three distinct zones basal layer, stratum spongiosum, and superficial stratum compactum. There is intense infiltration of leukocytes, synthesis of growth factors, decidualization of stromal cells, and a complete array of peptides and cytokines. All these changes are meant to receive and nurture the blastocyst.
IV. Menstrual phase
In the event of non-establishment of pregnancy, events including blood stasis, arteriolar spasm, tissue necrosis, ischemia and eventually enzymatic autodigestion occur which results in menstruation. Arterial and venous blood, remnants of endometrial stroma and glands, leukocytes, and red blood cells are all present in the menstrual flow along with superficial functional layer.
Join us in embracing the newer treatment modalities and advanced technology in the field of infertility. Get empowered by learning the intricacies and recent research in the basics of reproductive physiology and pathophysiology. Enrol in Fellowship in Reproductive Medicine at Medline Academics.