Vitrification of oocytes and embryos
Cryopreservation of human cells is an integral part of ART.
The results after oocyte vitrification have been extremely promising. A 2011 guideline from the ASRM and Society for Assisted Reproductive Technology (SART) indicated that mature oocyte vitrification and warming should no longer be considered as an experimental procedure because this technology is recommended in case of genotoxic therapy where there is lack of alternative options for fertility preservation.
Indications of oocyte freezing
- Laws of reproductive function due to chemotherapy, radiation, and oral surgery for cancer.
- Premature menopause and ovarian failure
- Overcoming ethical and legal issues involved in embryo freezing.
- Oocyte cryobanking for facilitation of egg donation programme.
- To prevent age-related infertility and options for delaying motherhood.
- Prevention of OHSS.
- Male factor infertility or inadequate seminal samples.
- Poor egg quality due to endometriosis.
- Immunological disorders.
Factors affecting oocyte survival
- Spherical shape and surface area : Oocytes are extremely difficult to cryopreserve because of their low surface area to volume ratio and high susceptibility to intracellular ice-formation. Perfect sphere of the oocyte slows down permeation and equal distribution of cryoprotectant in the oocyte. Concentration gradient results in a longer exposure of the outside to cryoprotectants which may lead to toxic damage in one part of the oocyte offering less than optimal protection in the other.
- Size : Size is a very important parameter. Larger the cell, more difficult it is to cryopreserve. Size of the oocyte affects crystal formation and the slow dilution or accumulation of toxic cryoprotectants thus increasing the challenge of survival.
- Mono cellular nature: Since the oocyte is a single-cell structure, there is no backup to regenerate from serious injuries as compared to multicellular embryo.
- Physiology of the membrane : It is very difficult to predict membrane permeability characteristics of the human oocytes.
- Cryoinjuries : The oocyte is highly susceptible to cryoinjuries to the cytoplasmic content and also the nuclear spindle. Chilling injury occurs at high temperature and induces irreversible damage to the cytosolic content, membrane, and zona pellucida. Hardening of the zona due to premature cortical granule release may result in decreased fertilisation rate. Therefore, a very careful approach has to be applied for cooling.
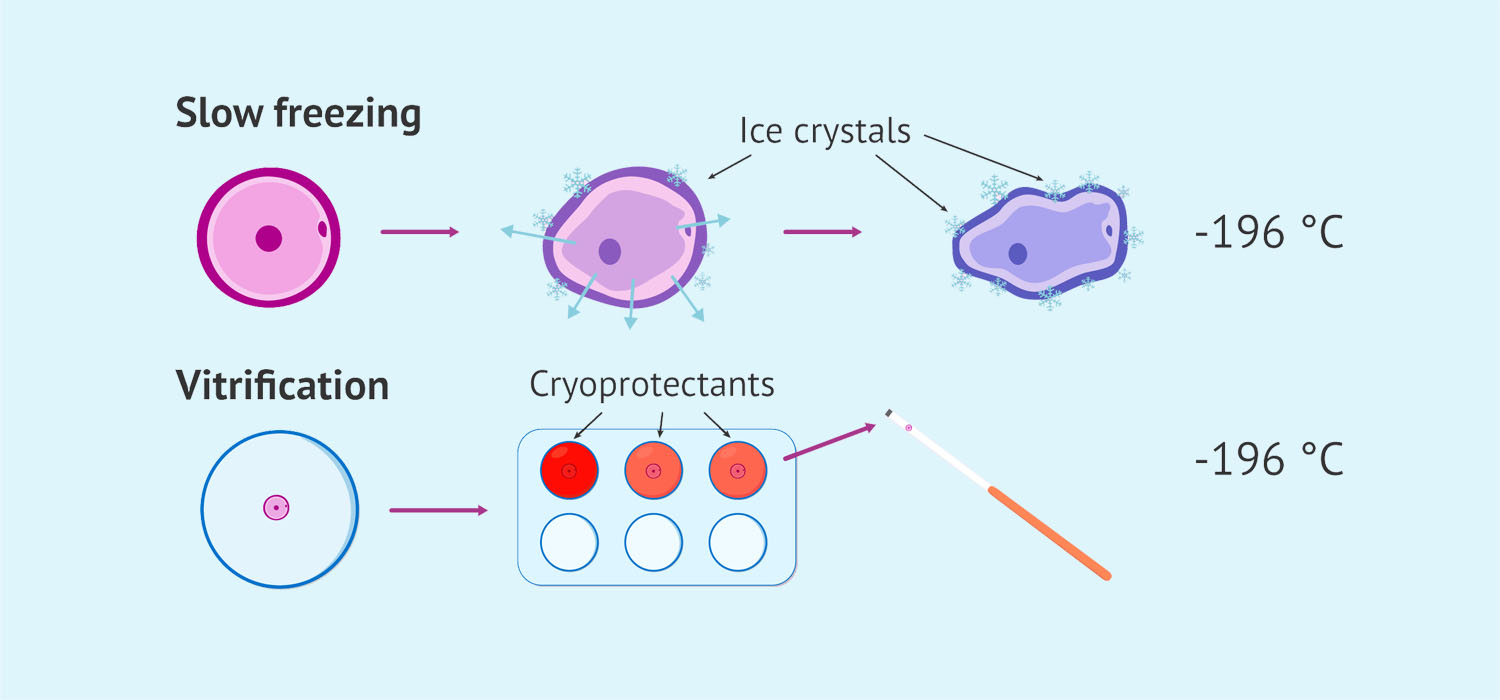
Vitrification
Vitrification is a process of cryopreservation which involves exposure of oocytes to high concentration of cryoprotectants and ultra rapid cooling to solidify the cell into a glass like state without the formation of ice crystals.
There are 5 principal steps in vitrification:
- Addition of cryoprotectants
- Cooling the cells to -196 degrees Celsius
- Storing at -196 degrees Celsius
- Warming the cells
- Removing the cryoprotectants
Cryoprotectants
There are mainly 2 types of crypto protectants.
1) Penetrating cryoprotectants
These penetrate the cell membrane. They protect the cells and tissues by replacing some of the water they contain, thus increasing the viscosity in the cells and preventing the formation of ice crystals. This makes the verification process easier by inducing the transition to glass state. The commonly used penetrating cryoprotectants are ethylene glycol, dimethyl sulfoxide or DMSO and propylene glycol.
2) Non penetrating cryoprotectants
The non penetrating cryoprotectants protect the cells from outside. They guard against osmotic damage, especially during the warming procedures. During the warming process, the transfer of oocytes from a solution containing a high concentration of cryoprotectant to an isotonic solution can lead to a reverse osmotic shock and swelling of the oocyte which may be dangerous. Use of a hypertonic solution containing non penetrating cryoprotectants can prevent this osmotic shock by slowing down the movement of water across the membrane. The typically used non penetrating cryoprotectants are sucrose and trehalose.
Freezing rate
High freezing rate is crucial to achieving proper vitrification and survival. So, this can be achieved in 2 ways. One is by direct contact between the sample and liquid nitrogen or there can be an indirect contact if the sample is contained in a closed carrier. The other way of increasing the freezing rate is to reduce the temperature of liquid nitrogen more rapidly. Heat transfer is achieved by a wider temperature difference, and it may also minimise the chances of formation of gas bubbles that can insulate the heat transfer. The liquid nitrogen temperature can be decreased by applying a vacuum over the liquid nitrogen or by the use of liquid nitrogen slush.
Currently the most acceptable target in designing Vitrification loading devices for oocytes or embryos is to use a small volume (less than one micro litre) of high concentration cryoprotectant (approximately 30%) and very rapid freezing rates of 15,000 to 13,000 degrees Celsius per minute.
Warming rates
The cells are more sensitive to warming rate compared to the cooling rate during vitrification. So slow warming is lethal for the cells since it allows formation of tiny ice crystals by recrystallization. To achieve high warming rates, it is essential that minimum volumes are used during vitrification. Mixing the cells in pre-warmed media will aid in achieving high warming rates.
Open versus closed system vitrification
In the open system vitrification, the cells come into direct contact with liquid nitrogen. However, in the closed system vitrification, the carrier is closed before plunging it into liquid nitrogen. There exist concerns about contamination and infection in the Open System Vitrification and this is overcome by:
- Proper sealing of carriers containing oocytes is an effective measure against contamination during storage.
- Alternative preventive step is filtration of liquid nitrogen and the application of accessory protective storage containers.
- Cross contamination may also be prevented by storing oocytes and embryos from patients with known infections in separate liquid nitrogen tanks.
Time schedule to be observed for oocyte vitrification and warming
Oocyte retrieval is done 34 hours after hCG injection. After another 2 hours, vitrification of the oocytes is done.
The oocytes are thawed 36 hours after hCG injection and after another 3 hours, ICSI is performed.
Effect of frozen thought who sides on meiotic spindle
The spindle is very sensitive to cryoprotectants and low temperature. However, these meiotic spindles are crucial for events following fertilisation during the completion of meiosis, the second polar body formation and migration of the prone nuclei. Incubation for one to 3 hours results in recovery of the spindles. Even though spindle abnormalities in cryopreserved oocytes were originally of concern, the incidence of chromosomal abnormalities in human embryos derived from cryopreserved oocytes is not significantly different from that of control embryos.
Safety of vitrification
There have been many concerns regarding the safety of vitrification and the metabolomics, proteomics and epigenetic risks involved with Vitrification. Transient changes in mitochondrial activity by vitrification can occur at the pre-antral stage but proteome of in vitro grown and matured oocyte is not affected. Oocyte proteome and developmental potential may get affected because of changes in cellular integrity and protein alterations with the use of high concentration of cryoprotectant. After studying animal models, it was observed that there is no increase in the risk of disturbances in spindle formation or chromosome segregation with vitrification of oocytes. Studies have revealed no difference in birth weight or congenital anomalies amongst those born from vitrified oocytes as compared to children conceived after fresh transfers.
Studies also suggests that there are no reported increases in miscarriages, chromosomal abnormalities and birth defects in the infants born from vitrified oocytes.
Hence, with robust techniques for oocyte vitrification, it has become easier to cryopreserve oocytes and also embryos. It has now become a part of Segmentation strategy for management of OHSS. As per the current technology available, open system vitrification is more efficient for oocyte vitrification and it is advised to vitrify the oocytes within 2 hours of retrieval and wait for 3 hours after warming before fertilising them. This helps to take care of the meiotic spindle recovery as well as avoids oocyte ageing. Oocyte vitrification does not increase the rate of aneuploidy or decrease the implantation potential of the embryos. Hence it is also a valuable tool for young women wanting to preserve their fertility for either medical or age-related reasons.
Conclusion
In the realm of Assisted Reproductive Technology (ART), the cryopreservation of human cells stands as a pivotal procedure, and within this domain, the vitrification of oocytes has emerged as a particularly promising technique. Highlighted in the 2011 guidelines from the ASRM and SART, mature oocyte vitrification has transcended its experimental status, finding recommendation especially in cases where genotoxic therapy threatens fertility with limited alternative options for preservation. The applications for oocyte freezing span a spectrum of medical scenarios, including safeguarding reproductive function amidst chemotherapy, radiation, and cancer surgeries, tackling premature menopause, facilitating egg donation programs, and addressing age-related infertility concerns. However, despite these advancements, challenges persist in ensuring optimal oocyte survival during the vitrification process, influenced by factors such as size, cellular physiology, and membrane characteristics. As medical professionals seek to delve deeper into the nuances of embryology and reproductive medicine, institutions like ours offer avenues for comprehensive learning and skill enhancement. For those keen on exploring the intricacies of oocyte vitrification and furthering their expertise in this domain, undertaking the Medline Academics fellowship in embryology course at our institution provides an enriching opportunity to delve into this specialized field.