Luteal Phase defect (LPD)
What is LPD?
LPD is a condition where the endogenous hormone production from ovaries is of insufficient quantity or duration to maintain a secretory endometrium for implantation and growth of embryo.
Clinically, when the luteal phase is less than 10 days, it can be termed as LPD.
Incidence of LPD has been variously quoted as 6.6 to 51%. Pr5evalance in normo-ovulatory primary/secondary infertility is 8.1% while it is around 32.5% in cases of recurrent miscarriage.
What causes LPD?
It is a misconception to presume that only luteal phase factors are responsible for LPD.
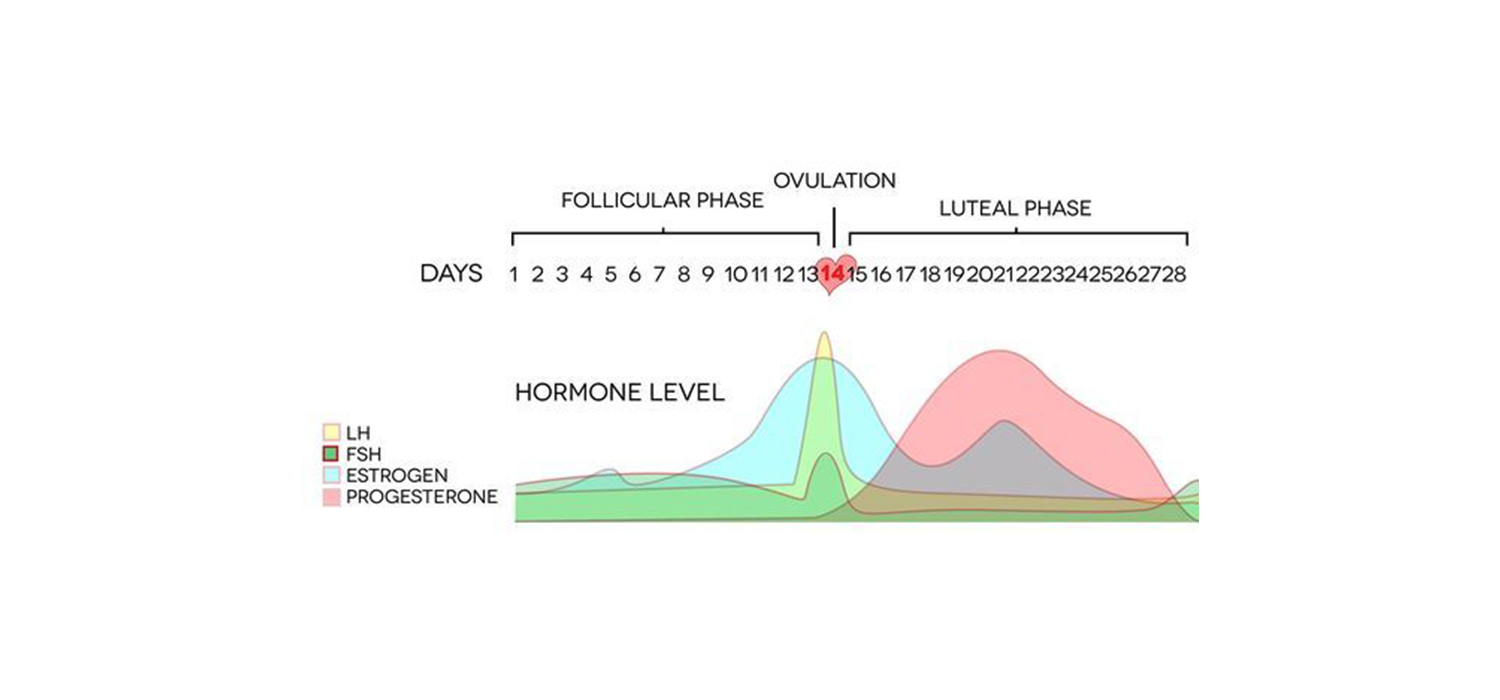
Follicular phase factors causing LPD include inadequate FSH/LH production, defective granulosa cell function, defective steroidogenesis, insufficient E2 production and priming of endometrium.
Luteal phase factors include inadequate LH surge, abnormal functioning of small/large luteal cells, insufficient progesterone production, reduced progesterone receptors on endometrium and abnormal hCG stimulus form endometrium.
What causes LPD in cycles with controlled ovarian stimulation (COS)?
Multi-follicular development in COS causing supraphysiological levels of estrogen causes LH suppression. Prolonged pituitary suppression in GnRH agonist cycles and a short duration of LH flare in GnRH antag cycles are other causes od LPD in COS. Destruction of granulosa cells destined to form corpus luteum during the process of oocyte retrieval in IVF/ ICSI cycles can also contribute.
Can LPD occur in natural cycles?
Yes. Excessive exercise, starvation, eating disorders, extremes of age, obesity, hyperinsulinemia are some conditions which can cause LPD. Endometriosis, thyroid/ prolactin disorders can contribute to LPD.
How is LPD diagnosed?
LPD is a clinical diagnosis (ASRM 2021). It is suspected when the luteal phase is less than 10 days. Women with short cycles, pre-menstrual spotting, unexplained infertility and first trimester pregnancy loss are more likely to have LPD.
A single reading of serum progesterone during luteal phase cannot be used for diagnosis as progesterone secretion varies form cycle to cycle and it is secreted in pulses. The sum of three mid-luteal progesterone (measured 5-9 days after ovulation) and exceeding 30 ngms/ml has a 100% specificity and 80% sensitivity in predicting LPD. However, this has not been validated and is not practical. So also, an endometrial biopsy and endometrial markers.
Colour dopplers of the corpus luteum (CL) can indicated LPD. The RI of CL correlates with plasma progesterone levels, as an index of luteal phase. Normally the RI of the follicle reduces after ovulation and continues to fall in late luteal phase. In LPD, there is a sustained increase of RI in luteal phase.
How is LPD treated?
Possible causes contributing to LPD like thyroid/ prolactin impairment, excessive stress, weight, exercises etc must be addressed and corrected.
The aim of correcting LPD include promoting endometrial maturation, enhancing its receptivity, and supporting implantation and early pregnancy.
Pharmacological treatments start with planning better stimulation protocols and ensuring good luteal phase support, often starting form the day of oocyte retrieval in cases of IVF/ICSI.
Since GnRH agonist trigger can cause a short luteal phase support, dual trigger is employed. This involves using a small dose of hCG (1500 IU) along with the agonist trigger.
Progesterone supplementation has been found to be effective, irrespective of the route of administration. It is started on the evening of oocyte retrieval and has to be continued for the next 3 days, though the general practice is to continue progesterone supplementation till 8 weeks of pregnancy. Inj hCG, acting as a surrogate for LH, helps to prevent luteal phase deficiency. It stimulates the production of estrogen and progesterone from the corpus luteum. However, the risk of OHSS precludes its use routinely.
GnRH agonist is also used to prevent LPD. It increases the LH, has a positive effect on endometrial receptors and also a direct effect on the embryo. It has shown better results when used with progesterone than when progesterone was used alone.
Is Luteal phase support (LPS) needed in IUI?
LPS is beneficial when used in those cycles where gonadotropins have been used for ovarian stimulation. Clinical pregnancy rates and live birth rates have been better with progesterone supplementation. There is no role of LPS in cycles where clomiphene citrate was used for ovulation induction.
Also, there is no role of progesterone support in unstimulated or natural cycles.
How is LPS given in FET (Frozen Embryo Transfer) cycles?
FET cycles merit exogenous estrogen and progesterone supplementation. It is given after embryo transfer.
For more info, Follow : medlineacademics.com